
Is HIPAA International? Comparing Global Data Protection Standards
HIPAA, or the Health Insurance Portability and Accountability Act, is a U.S. law designed to protect patient health information. While it is not an international regulation, it is international in its potential to impact how international entities handle Protected Health Information (PHI) for U.S. patients. This article will explore HIPAA’s jurisdiction, its implications for international business associates, and how it compares to other global data protection standards.
Key Takeaways
• HIPAA is primarily a U.S. regulation that protects the privacy and security of Protected Health Information (PHI), with compliance obligations extending to international business associates handling PHI.
• Significant differences exist between HIPAA and GDPR, including consent requirements, the right to data deletion, and breach notification timelines, highlighting the need for tailored compliance strategies in global healthcare.
• Effective HIPAA compliance abroad demands a comprehensive approach, including regular risk assessments, secure data transmission practices, and the appointment of Data Protection Officers to oversee compliance efforts.
Understanding HIPAA’s Jurisdiction
HIPAA, the Health Insurance Portability and Accountability Act of 1996, is a key U.S. healthcare regulation that oversees the privacy and security of Protected Health Information (PHI) domestically. Enforced by the Office for Civil Rights in the Department of Health and Human Services, HIPAA establishes strict guidelines for healthcare providers, health plans, and healthcare clearinghouses regarding the management of PHI.
Covered entities, which include health plans, healthcare providers, and healthcare clearinghouses, are at the core of HIPAA compliance. These entities are responsible for ensuring that PHI, which encompasses any information related to an individual’s health status, provision of healthcare, or payment for healthcare, including individually identifiable health information, is securely managed and protected. The HIPAA Privacy Rule outlines explicitly the conditions under which PHI can be disclosed without patient consent, ensuring that the privacy of health information is maintained.
The jurisdiction of HIPAA, however, is primarily confined to the United States. Although HIPAA regulations apply to entities operating within the U.S., the global nature of modern healthcare requires an understanding of how these rules interact with international data protection standards. This brings us to the role of international business associates in HIPAA compliance.
International Business Associates and HIPAA
Business associates are vital in the global healthcare landscape. These entities, including various contractors and vendors, handle PHI for covered entities and must adhere to HIPAA regulations, even when operating outside the United States. This extension ensures PHI protection regardless of its location.
International business associates must formalise this compliance by entering into a Business Associate Agreement (BAA) with the covered entities they serve. These agreements stipulate that business associates will safeguard PHI according to HIPAA standards and outline specific responsibilities, such as not using PHI for any purpose other than those agreed upon and ensuring that any subcontractors also adhere to HIPAA regulations.
For example, an international IT service provider managing electronic PHI for a U.S. healthcare organisation must have a signed Business Associate Agreement in place. This agreement ensures that both parties know their roles and responsibilities in maintaining HIPAA compliance. Moreover, it underscores the importance of conducting regular risk assessments and implementing strict access controls to prevent data breaches.
For international business associates, HIPAA compliance extends beyond signing agreements; it requires a deep understanding of the regulations and a commitment to PHI protection. Adhering to these standards helps them avoid severe penalties and maintain compliance.
HIPAA vs. GDPR: Key Differences
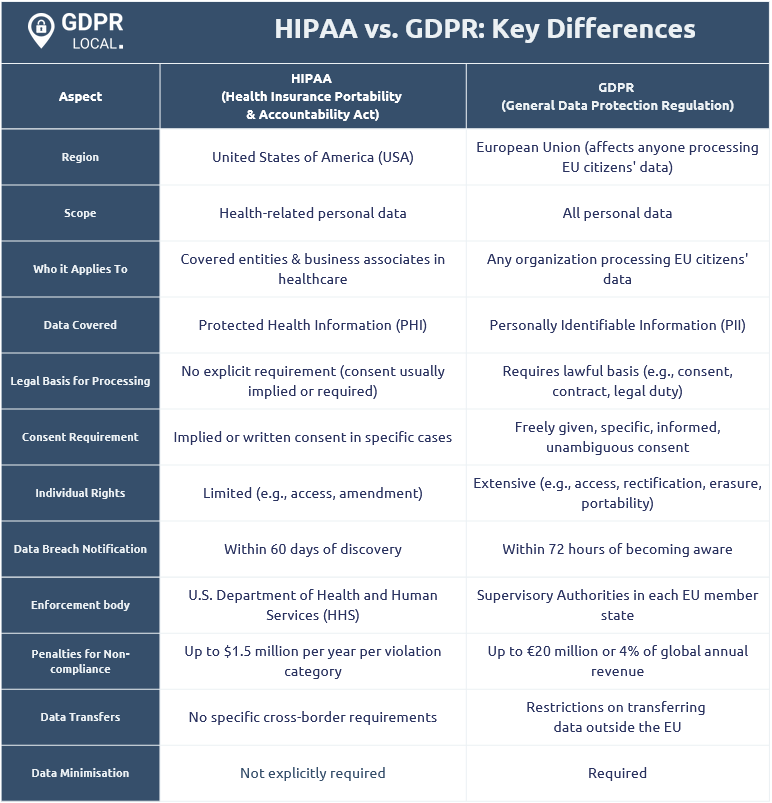
HIPAA and GDPR are two major data protection frameworks with distinct scopes and requirements. HIPAA targets healthcare entities within the United States, whereas GDPR applies to any organisation processing the personal data of individuals in the EU or UK, covering a broader jurisdiction.
A key difference between HIPAA and GDPR is the right to data deletion. GDPR grants individuals the right to request data deletion, known as the “right to be forgotten.” In contrast, HIPAA does not permit medical record deletion, focusing on preserving and protecting health information.
Consent requirements also differ significantly. GDPR mandates explicit consent for any use of personal data, requiring organisations to obtain explicit and affirmative permission from individuals before processing their data. In contrast, HIPAA allows healthcare providers to disclose protected health information without patient consent under certain circumstances, such as treatment, payment, and healthcare operations.
Breach notification timelines further distinguish these regulations. GDPR mandates reporting all data breaches to relevant authorities within 72 hours to ensure swift action. Conversely, HIPAA permits up to 60 days to notify affected individuals of breaches involving 500 or more people, offering a more lenient timeline.
HIPAA Compliance for Global Healthcare Providers
Global healthcare providers encounter unique challenges in maintaining HIPAA compliance. Regardless of size or location, these entities must adhere to HIPAA regulations to safeguard U.S. citizens’ privacy rights and avoid legal repercussions, especially as patient data frequently crosses borders in modern healthcare.
For example, an international company providing cloud services to a U.S. healthcare provider must implement robust security measures to protect PHI. Similarly, pharmaceutical firms conducting U.S. clinical trials must follow HIPAA standards when handling participants’ health information. These examples highlight the need for due diligence in ensuring international partners comply with HIPAA standards.
A comprehensive set of policies and procedures is essential for adhering to HIPAA’s privacy and security rules. This includes regular training for all employees and independent contractors to ensure they understand and comply with HIPAA requirements. Additionally, appointing designated compliance leaders, such as a Privacy and Security Officer, is a best practice for maintaining compliance.
Regular audits and risk assessments are also critical in identifying and mitigating vulnerabilities in handling electronic PHI. By implementing these practices, global healthcare providers can ensure they remain HIPAA compliant and continue to protect patient data effectively.
Handling Cross-Border Data Transfers
Cross-border data transfers present significant challenges and risks for healthcare organisations handling PHI. Ensuring compliance with HIPAA during these transfers is crucial to protect patient privacy and maintain regulatory adherence. As data moves across borders, the potential for security breaches increases, making it essential to adopt stringent safeguards.
Using secure data transmission protocols is essential to protect PHI during international transfers. These protocols encrypt and securely transmit data, minimising unauthorised access risks. Additionally, implementing physical and technical safeguards can further enhance cross-border data security.
International partners must also adopt HIPAA-compliant practices to mitigate risks associated with handling PHI. This includes regular risk assessments, adherence to Business Associate Agreements, and implementing robust security measures. By taking these steps, healthcare organisations can ensure that PHI remains protected, regardless of where it is transferred or stored.
Case Study: A Global Health Organization’s HIPAA Compliance Journey
Take the example of a global health organisation that successfully navigated HIPAA compliance complexities while managing U.S. patient data. This organisation faced challenges such as aligning international data practices with HIPAA requirements and ensuring all employees received adequate HIPAA training.
A major hurdle was integrating HIPAA compliance into their existing data protection framework. This required comprehensive risk assessments, detailed policies and procedures, and the appointment of compliance leaders to oversee the process and ensure alignment with HIPAA and international standards.
Despite the challenges, the organisation significantly improved its data protection practices. Regular audits and continuous training kept staff informed about HIPAA requirements and responsibilities. Advanced security measures were also implemented to protect electronic PHI, enhancing compliance efforts.
The outcome of this journey was a robust framework for ongoing HIPAA compliance and enhanced data protection practices. This case study is a valuable example for other global healthcare organisations striving to achieve HIPAA compliance.
Practical Steps for Ensuring HIPAA Compliance Abroad
Ensuring HIPAA compliance abroad requires a strategic approach and a commitment to continuous improvement. International businesses should conduct regular risk assessments to identify vulnerabilities in data-handling practices. These assessments are particularly crucial for remote workers accessing sensitive data from various locations.
A Virtual Private Network (VPN) is essential for remote workers to transmit sensitive patient data securely. VPNs provide an additional layer of security, ensuring that data remains encrypted and protected during transmission. Additionally, establishing strict policies on the use of personal devices can significantly enhance security and compliance in a remote work setting.
Developing a comprehensive compliance program tailored to protect sensitive health data is essential for international businesses. This program should include detailed policies and procedures, regular employee training, and designated compliance leaders. Implementing these measures ensures HIPAA compliance and adequate patient data protection.
Continuous education and policy updates are crucial to adapting to evolving regulations and emerging threats. Regular training sessions and updates ensure all staff members know the latest compliance requirements and best practices. These practical steps help international businesses achieve and maintain HIPAA compliance.
The Role of Privacy and Security Officers in HIPAA Compliance
HIPAA does not explicitly require a Data Protection Officer (DPO) but mandates the designation of a Privacy and Security Officer. These roles are essential for managing HIPAA compliance across the organisation. The Privacy Officer handles policies and procedures for using and disclosing protected health information (PHI).
At the same time, the Security Officer focuses on protecting electronically protected health information (ePHI) through technical and administrative measures. Both roles work together to conduct risk assessments, implement safeguards, and ensure staff receive ongoing HIPAA training. They also coordinate responses to potential data breaches and communicate with affected parties when necessary.
Assigning clear responsibilities to these officers strengthens compliance efforts and supports a proactive approach to data privacy and security in the healthcare environment.
Summary
Navigating the intricacies of HIPAA compliance, especially on a global scale, requires a thorough understanding of its jurisdiction, the responsibilities of international business associates, and the key differences between HIPAA and other data protection regulations like GDPR. By taking practical steps such as conducting risk assessments, implementing secure data transmission protocols, and appointing dedicated compliance leaders, healthcare organisations can ensure they remain compliant and protect patient data effectively.
The case study of a global health organisation highlighted the challenges and successes in achieving HIPAA compliance, providing valuable insights for other entities in similar situations. The role of Data Protection Officers is crucial in this journey, as they oversee compliance efforts and ensure that all staff members are adequately trained and informed.
In conclusion, understanding and adhering to HIPAA regulations is essential for any healthcare provider or organisation handling U.S. patient data, regardless of location. By staying informed and proactive, organisations can protect health information and maintain compliance in an ever-evolving regulatory landscape.
Frequently Asked Questions
Is HIPAA applicable in the EU?
HIPAA does not apply in the EU, as it is a US law. Instead, the General Data Protection Regulation (GDPR governs personal data protection for individuals in the EU.
Does HIPAA apply outside the US?
Yes, HIPAA can apply outside the US if the covered entity or business associate enters into a business associate agreement and complies with HIPAA requirements.
Does HIPAA apply to the UK?
HIPAA does not directly apply in the UK; however, US private providers operating there must comply with HIPAA. The National Health Service implements its own security policies for public health services in England, Wales, and Scotland.
Does HIPAA apply to international companies?
Yes, HIPAA applies to international companies that manage U.S. citizens’ Protected Health Information (PHI). It requires them to comply with HIPAA regulations and enter into Business Associate Agreements. Compliance is essential to ensure the protection of sensitive health information.
What are the key differences between HIPAA and GDPR?
The key differences between HIPAA and GDPR lie in their scope and requirements. GDPR applies to all organisations handling the personal data of EU or UK citizens, mandates explicit consent, and requires breach notifications within 72 hours. In contrast, HIPAA is limited to U.S. healthcare entities, does not permit data deletion, and allows up to 60 days for breach notifications. This highlights the varying levels of privacy protections and obligations under each regulation.